FUNDED BY
THE WELLCOME TRUST

OVERVIEW
The vision of the Oxford Centre for Integrative Neuroimaging (OxCIN) is to bridge the gap between laboratory neuroscience and human health. Neuroimaging is a powerful tool to build bridges between these levels of investigation. It provides measurements that are sensitive to cellular phenomena and can be acquired not only in humans, but also in animal models, which in turn can be complemented with precise mechanistic investigations. In the Preclinical Imaging Group we aim to develop novel methods to acquire and analyze rodent MR images, and combine this imaging data with cellular and molecular probes to provide cross-scale insights into the function and structure of the brain. We use sophisticated experimental designs consisting of advanced genetic models, circuit manipulations, and behavioural assays to span topics from molecular to cognitive to clinical neurosciences. Our work is tightly coupled to the rest of OxCIN, with a particular emphasis on translating the insights generated from our preclinical studies back to humans.
imaging data examples
3D Mouse Brain Rendering from MRI
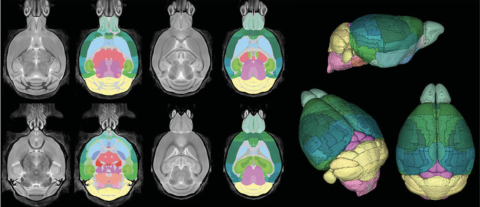
Mouse Brain Atlas
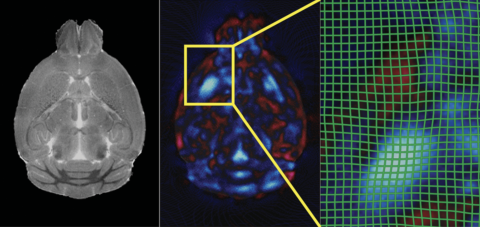
Deformation Based Morphometry
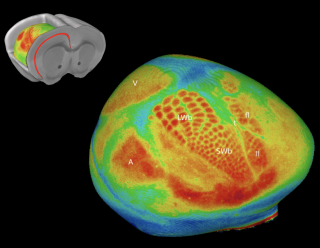
Optical Imaging of Barrel Cortex
DPHIL PROJECTS / RESEARCH PLACEMENTS
To learn more about available placements within the Preclinical Imaging Group email: oxcin.preclinical@ndcn.ox.ac.uk and send your CV as well as a letter of intent. Please explain clearly what your background is and what kind of research you are interested in. More information can be found here.
RESEARCH PROJECTS
Coupling neuroimaging with CLARITY and single cell genomics to dissect sex differences in the developing brain.
Project lead: Jason Lerch
Many neurobehavioral diseases affect females and males differently, and emerge at different stages of life, leading to the conclusion that one sex is protected from diseases by inherent sex factors, such as gonadal hormones and sex chromosomes. The sites and timing of these effects on brain development are unknown. The method of high-throughput high precision whole-brain MRI imaging has the power to detect such changes with great sensitivity, in both animal models and humans. This project will exploit these powerful methods to separate gonadal hormonal and sex chromosome effects in the mouse model “Sex Chromosome Trisomy” (SCT), at 9 different ages of development, to discover where and when these factors cause sex differences in brain development. The SCT model compares mice with different numbers and types of sex chromosomes (XX, XY, XXY, XYY), each genotype present in gonadal males or females. Then, a novel pipeline of analysis will compare mouse and human brain development at many stages and in informative groups (differing by age, sex, hormonal status, and sex chromosome complement) to determine which changes in mouse brain are also found in humans, in specific brain regions related to different diseases. The analysis will point to changes in mouse brain that model human brain development. These studies will also pinpoint new sex-biasing effects of hormones and sex chromosomes at localized brain regions at specific developmental stages in mice, leading to further investigation of cellular and molecular changes caused by sex at those sites. Cellular effects (cell size, number, density of defined cell populations) of sex-biasing factors will be measured using CLARITY. Gene pathways responding to hormonal and sex chromosome effects in individual cell types in specific brain regions will be measured using single cell RNA-seq. These studies, combining high-resolution neuroimaging, CLARITY, and single cell sequencing, will provide a foundation of concepts about where in the brain, and when during development, specific cell populations respond to sex factors, as a prelude for hypothesizing which sex differences underlie the protective effects of sex-biased factors in cells.
This project is funded by the National Institutes of Health in the United States and is led by Jason Lerch (WIN/Oxford), Art Arnold (UCLA), Armin Raznahan (NIH), Allan MacKenzie-Graham (UCLA), and Xia Yang (UCLA).
Promoting and monitoring brain recovery after stroke
Project lead: Kamila Szulc-Lerch
Stroke is one of the leading causes of motor and cognitive disability in humans. Even though the brain damage induced by a stroke is often irreversible, some degree of recovery is frequently observed as a consequence of functional and structural reorganization of the spared brain tissue. These small improvements in post-stroke function matter to the overall quality of life of patients, and there is a great need to come up with more effective strategies to improve outcomes. The overarching goal of this project is to use mouse models of stroke to identify novel, safe and effective strategies of enhancing functional post-stroke recovery (motor and cognitive) in mice that have translational potential and could benefit human patients recovering from stroke. We aim to test different treatments including pharmacological, lifestyle, electro-magnetic stimulation and/or combinations thereof while using advances in mouse genetics to carefully control genetic and hormonal effects of sex on injury and brain recovery.
This project is funded by the WIN Seed Grant and is led by Kamila Szulc-Lerch (WIN/Oxford) in collaboration with Yvonne Couch (Radcliffe Department of Medicine) and Tracy Farr (Nottingham University).
Fingerprinting mood disorders of metabolic origin
Project lead: Antoine Cherix
Insulin resistance has been recently put forward as a potential link between metabolic deregulations, typically observed in metabolic syndrome (MeS), and depression. MeS has a high co-occurrence with depression and could thus represent an etiological factor for a large subset of patients with mood disorders.
The goal of this project is to study how insulin signaling regulates brain metabolic homeostasis and impacts depressive behavior. By combining neuroimaging, molecular analyses and behavioral measurements in mice and humans, this project aims at identifying translatable neuroimaging markers related to brain insulin hypofunction. In particular, this project focuses on how magnetic resonance spectroscopy (MRS) can provide a useful fingerprint of neuroenergetic impairments in brain structures of depressed patients.
This project is funded by a Postdoctoral Mobility Fellowship (Antoine Cherix) from the Swiss National Science Foundation (SNSF)
There was an error while rendering this tile
There was an error while rendering this tile